Fe Cn 6 4- Molecular Orbital Diagram
Hybridization hybridisation coordination compounds atomic octahedral Molecular orbital diagram cn How to find the oxidation number for fe in the fe(cn)6 4- ion.
[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why
Coordination magnetic potassium properties ferrocyanide chemistry electrons fe cn diagram spin field iron crystal low high unpaired orbitals complexes compounds [fe(cn)6]4– is diamagnetic while [fef6]4– is strongly paramagnetic. why Orbital molecular k4 xas k3 studies
Potassium ferrocyanide
Write the hybridization and shape of [fe (cn)6]3— (atomic number of feOxidation ion Introducing complex ionsIon complex bonding orbital diagram electrons metal fe3 coordination ions iii structure ii level 3d chemistry libretexts complexes introduction al.
Molecular orbital diagram for cnHybridization orbital complex electron unpaired doubts regarding ask Orbital configuration electronWrite the hybridization and shape of [fe (cn)6]3— (atomic number of fe.

Fe4[fe(cn)6]3: a cathode material for sodium-ion batteries
Zigya diamagnetic fef6 strongly paramagnetic hence pairing ligandFe orbital diagram Cn molecular orbital diagramOrbital molecular fe diagram cn spin high diagrams 1g schematic.
Orbital partial fluorescence yield absorption occupied orbitals homo 4aSpectroscopic and magnetic properties of coordination compounds Potassium ferrocyanide.

![Fe4[Fe(CN)6]3: a cathode material for sodium-ion batteries - RSC](https://i2.wp.com/pubs.rsc.org/en/Content/Image/GA/C4RA07531E)
Fe4[Fe(CN)6]3: a cathode material for sodium-ion batteries - RSC

Cn Molecular Orbital Diagram - Drivenheisenberg
![Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe](https://i2.wp.com/s3mn.mnimgs.com/img/shared/ck-files/ck_57d508ccd7d91.png)
Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe

introducing complex ions - ligands and bonding
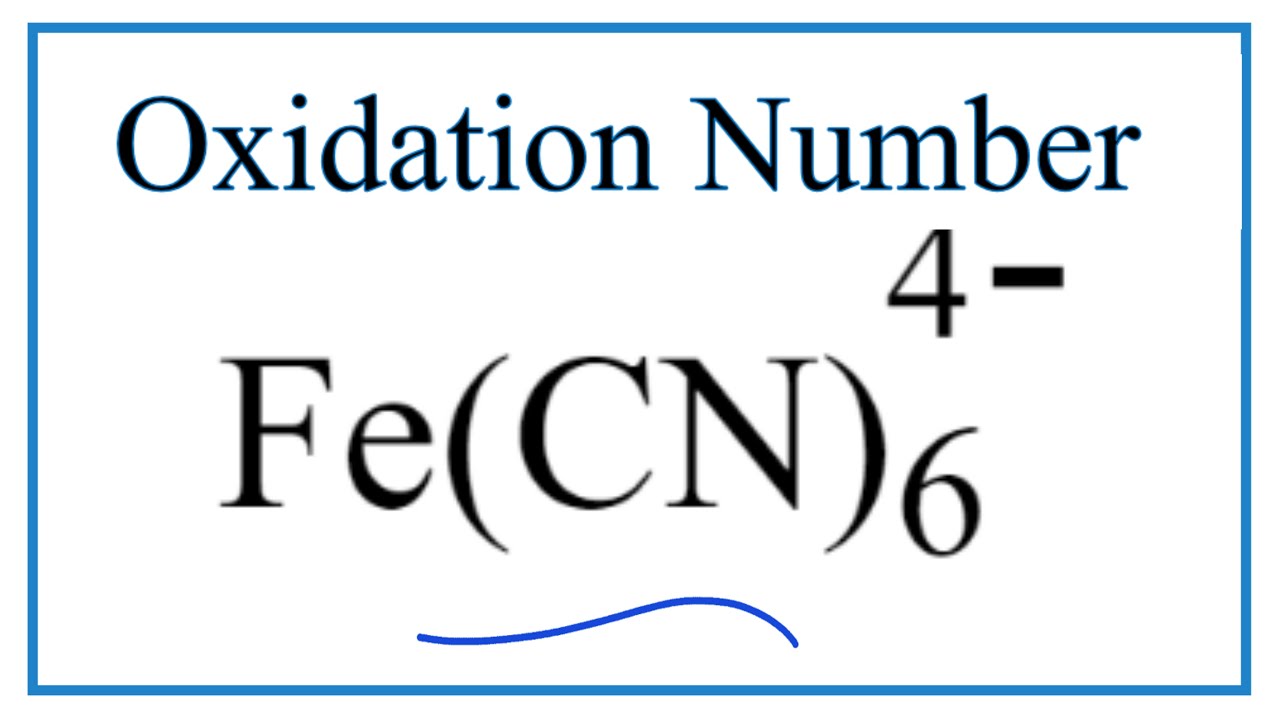
How to find the Oxidation Number for Fe in the Fe(CN)6 4- ion. - YouTube

Potassium Ferrocyanide - Structure, Properties & Uses of K4Fe(CN)6
![[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why](https://i2.wp.com/www.zigya.com/application/uploads/images/chen12070386_571486ba519b3.png)
[Fe(CN)6]4– is diamagnetic while [FeF6]4– is strongly paramagnetic. Why
![Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe](https://i2.wp.com/s3mn.mnimgs.com/img/shared/ck-files/ck_57d50a54db98e.png)
Write the hybridization and shape of [Fe (CN)6]3— (Atomic number of Fe

Molecular Orbital Diagram For Cn - Wiring Site Resource

Spectroscopic and Magnetic Properties of Coordination Compounds