Octahedral Molecular Geometry Polarity
Geometry molecular molecule octahedral coordination chemistry chemwiki libretexts coordinated shape figure structure chem1 planes Octahedral geometry molecular Molecular geometry lone pairs geometries bonding model chemistry shapes molecules vsepr covalent models basic electron bent chem theory linear vsper
3.4.1: Molecular Shape - Chemistry LibreTexts
Molecular trigonal bipyramidal pyramidal structures tag How is "co"_2 nonpolar? + example Structure geometry molecular chemistry pairs atoms electron shape pair bonds density chem angle vsepr polarity regions lone geometries around two
9.7: the shapes of molecules
Vsepr theory octahedral shape hexafluoride ppt sulfur sf6 powerpoint presentation linear need know do not slideserve3.4.1: molecular shape Trigonal pyramidal molecular geometry archivesHow do you determine if a molecule is planar? : r/mcat.
Geometry chemistry planar molecule molecular shape trigonal examples chart determine axe bipyramidal nonpolar if octahedral chemical each chem vs exampleStructure geometry molecular chemistry theory geometries atoms electron chem shape polarity pair pairs bonds density angle vsepr regions around region Molecular electron octahedral trigonal bipyramidalMolecular geometry.

Molecular structure and polarity
9.2: valence bond theoryMolecule planar determine do chart if mcat 20for jpeg trigonal examples bipyramidal octahedral which following example courses 1941 west amazonaws Molecular geometryChemistry hybridization orbitals hybrid shape bond theory table valence shapes molecular atomic central vsepr atoms sp orbital electron sp2 molecules.
Ch. 9 molecular geometry .


Molecular Structure and Polarity | CHEM 1305 Introductory Chemistry

9.7: The Shapes of Molecules - Chemistry LibreTexts

9.2: Valence Bond Theory - Chemistry LibreTexts

3.4.1: Molecular Shape - Chemistry LibreTexts

PPT - VSEPR Theory PowerPoint Presentation, free download - ID:1157150

Ch. 9 Molecular Geometry

Molecular Geometry
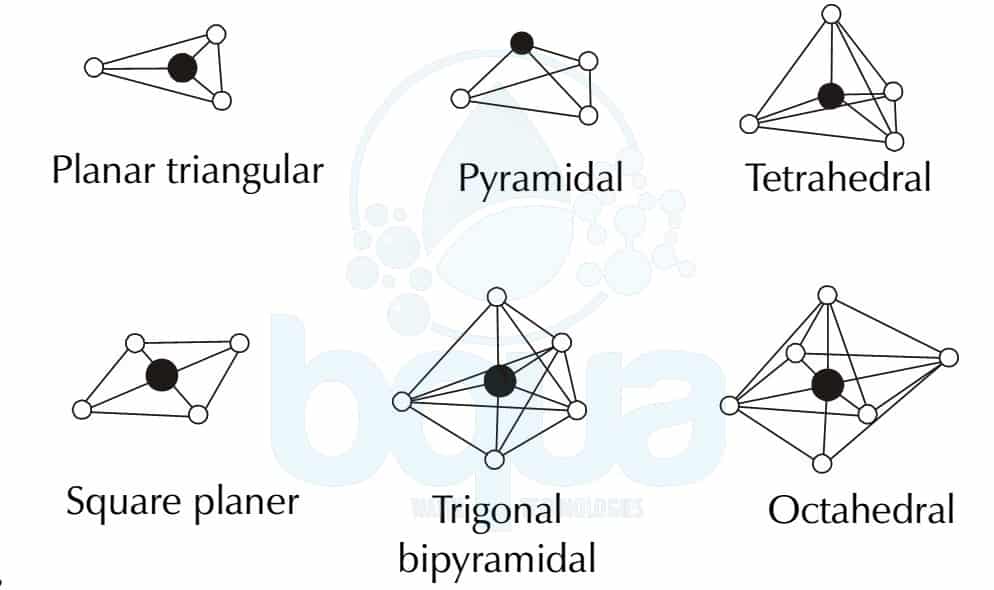
Trigonal Pyramidal Molecular Geometry Archives | BQUA

How is "CO"_2 nonpolar? + Example